Developing a nanotechnology-enabled glaucoma device

VisiPlate is designed to treat open-angle glaucoma
VisiPlate is an investigational implant designed to lower intraocular pressure in patients with open-angle glaucoma
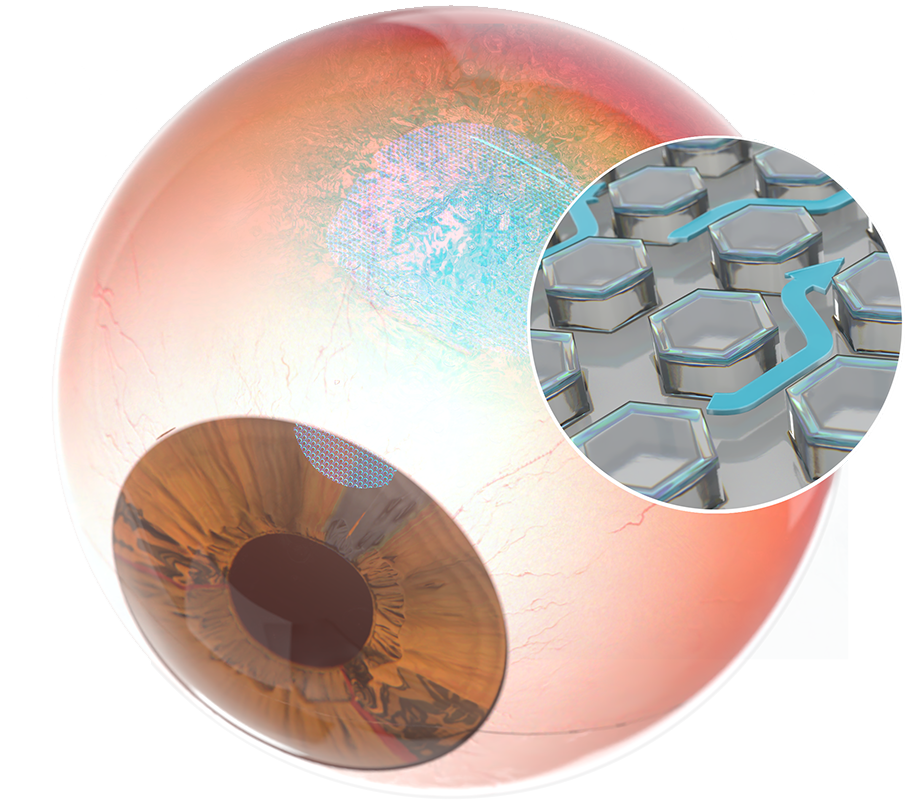
A next-generation aqueous shunt
Multichannel – VisiPlate is designed to lower eye pressure through a network of microchannels that control aqueous drainage from the anterior chamber.

A next generation aqueous shunt
Ultrathin – VisiPlate is several times thinner than a human hair. VisiPlate leverages an ultrathin material with the goal of achieving greater patient comfort and aesthetics.
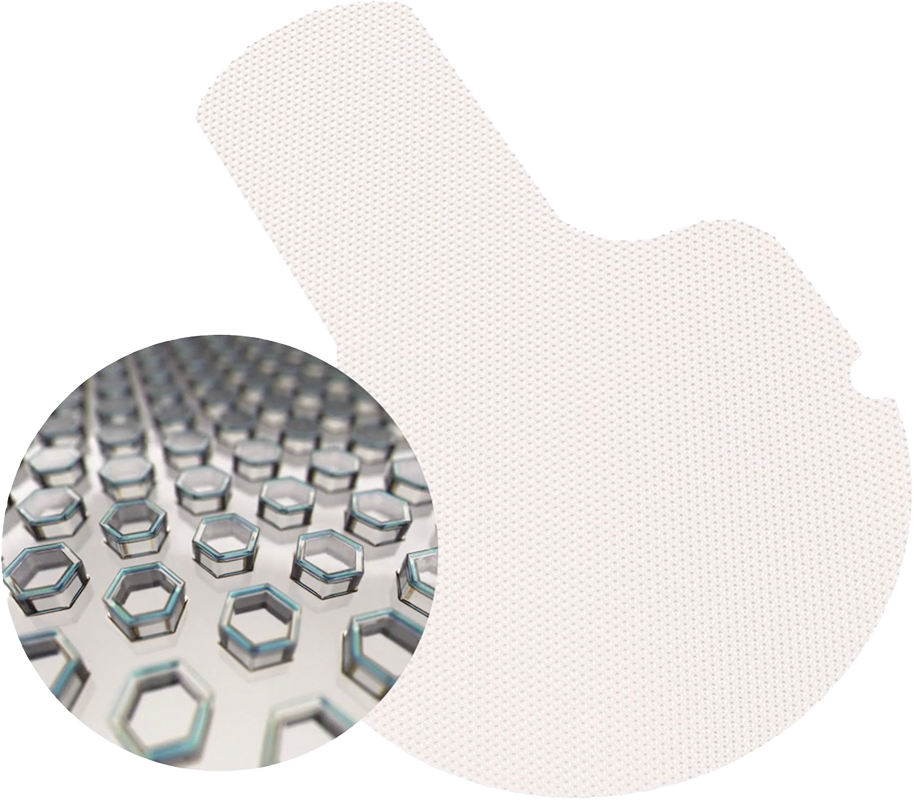
A next-generation aqueous shunt
Innovative Composition – VisiPlate is composed of alumina and Parylene-C with the goal of minimizing foreign body response and reducing the risk of scarring.
Building at the cutting edge
Avisi leverages MEMS (Micro-Electro-Mechanical Systems) manufacturing and utilizes high-performance equipment and advanced micro-fabrication manufacturing techniques. This development of small and advanced medical devices is intended to be at the forefront of minimally-invasive treatments.
Avisi’s proprietary material technology features an ultrathin, freestanding material, invented at the University of Pennsylvania.
Avisi’s scientific advisors hail from world-class institutions and bring together a diverse array of expertise. Drawing from leading universities, renowned research centers, and top-tier industry leaders, their experience helps guide Avisi.
Glaucoma is the "silent thief of sight"

Glaucoma is the leading cause of irreversible blindness. It was estimated to affect 4 million in the US and 80 million world-wide in 2020. The overall prevalence within the US is 2.1% and 3.54% globally. It is estimated that 3.36 million people 40 years and older in the US have open-angle glaucoma. This disease is on the rise and expected to reach 111.8 million globally by 2040.

Glaucoma is a group of eye diseases that damage the optic nerve and result in vision loss. The two most common types are primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). 90% of US glaucoma patients have POAG. There are typically no early warning signs or symptoms for POAG which is why it is known as the “silent thief of sight.” Consequently, routine eye exams are critical to diagnosis. No cure for glaucoma has been discovered yet and the only clinically proven way to modify disease progression is to lower the pressure inside the eye. Current treatments cannot reverse vision loss but are designed to slow disease progression, thereby preventing further vision loss.
Glaucoma is the "silent thief of sight"

Everyone is at risk for developing glaucoma, but certain groups have a higher risk. All people over the age of 60 are 6 times more likely to develop a type of glaucoma. Those with diabetes are twice as more likely to develop a type of glaucoma. Prevalence also varies with race. People of African-Caribbean and African American descent over the age of 40 are 8 times more likely to develop POAG.

Today, the national economic impact of glaucoma takes up the majority of $5.8 billion spent on treatment and management of optic nerve disorders. Ten million doctor visits occur each year for glaucoma resulting in $2.5 billion in annual healthcare costs. The burden of these costs on the US healthcare system is expected to significantly rise as the number of patients increases. Only 50% of people with glaucoma are diagnosed. The other 50% are unaware of their disease. Additionally, it is projected that the number of people affected by glaucoma will increase two folds by 2050.
Avisi in the news

Avisi Technologies Shortlisted for Junkosha Innovator of the Year Award
Avisi Technologies has been shortlisted for Junkosha’s second-year Innovator of the Year Award program. This year had a tremendous number of applicants with Avisi Technologies being 1 of the 11 shortlisted to impress the panel of expert judges chosen for their vast industry experience in medical devices. The winning company will be awarded a $25,000 […]

Healthtech Leader of the Month
Avisi, a trailblazing medical device company, is developing VisiPlate, a nanotechnology-enabled aqueous shunt designed to treat glaucoma. Working closely with ophthalmologists and experts, Avisi is committed to introducing a reliable, safe and effective solution for glaucoma patients.
Money Moves: Avisi Technologies raised a seed round
The company — a 2020 RealLIST Startups honoree — aims to treat open-angle glaucoma with an ocular implant that’s designed to remove excess fluid from inside the eye. Its recent funding injection totals just over $4 million.
Supported by